Interventions
This page is dedicated to documenting my interventions through a first-person perspective.
This page is dedicated to documenting my interventions through a first-person perspective.
Sweating is the body's natural cooling system, preventing overheating through evaporation. When body temperature rises, the eccrine glands release a clear, odorless fluid, which absorbs heat as it evaporates from the skin. In contrast, apocrine glands, found in the armpits and groin, produce a thicker fluid that, when mixed with bacteria, causes body odor.
Despite common myths, sweat does not remove toxins, it is composed of 99% water, along with small amounts of salt, proteins, carbohydrates, and urea. Detoxification happens in the kidneys and liver, not through sweating.
Hyperhidrosis occurs when eccrine glands overact, producing excessive sweat beyond what is necessary. It can be primary (localized and often hereditary) or secondary (linked to medical conditions or medications), significantly impacting daily life and comfort.
Sweat is often seen as something uncomfortable, a reaction to heat or exertion that we try to hide or control. But what if sweat is more than just a bodily function? What if it holds insights into how we react to our environment, to social pressure, to movement, and to our own self-awareness?
In this intervention, I conducted multiple experiments to explore the factors that trigger my sweat, how it manifests in different situations, and how my perception of it changes depending on context. Through these experiments, I aimed to answer key questions:
Does sweat respond more to physical effort or mental state?
How does environment (temperature, ventilation, crowd density) affect sweat production?
What role does social perception play in my discomfort or acceptance of sweat?
Can external tools (like a fan or clothing) change my experience with sweating?
EXPERIMENT 1: CARDIOVASCULAR ACTIVITY (Climbing a hill)
Context: I exposed myself to moderate cardiovascular exercise by climbing a hill in the city. The goal was to see how my body reacted to steady movement, temperature changes, and wind exposure.
Questions:
What are the first areas of my body to start sweating?
How does wind affect my perception of sweat?
Is sweat linked more to heat or to physical exhaustion?
Findings:
Sweat first appeared in my armpits, then my head, and finally my lower back.
I felt cold rather than hot while sweating, as the wind made the moisture on my skin feel chilling.
Entering the gym after the climb created an instant feeling of heat and discomfort, showing how sudden temperature shifts amplify sweat perception.
Does hydration impact where and how much I sweat? If I drink more before exercise, will it increase or regulate my sweating?
How does a sudden change in environment (from cold outdoors to a warm gym) impact my sweat response?
Would I sweat differently if I gradually adjusted to temperature changes instead of transitioning abruptly?
Can focusing on a specific task reduce or block sweating?
Does an intense sensory experience (loud music, lights, body contact) make sweat feel less important?
How does the level of trust with people around me influence my sweating and discomfort?
Is sweat more noticeable in moments of direct exposure (when someone talks to me) or in moments of uncertainty (when I don’t know what to do)?
Does speaking confidently reduce sweat levels? If I know exactly what to say, will I sweat less?
How does my experience change if I use different tools to control sweat (fan, towel, absorbent clothing)?
Does the type of music or dancing speed influence my perception of sweat?
How does the perception of sweat change when I hide it vs. when I intentionally show it?
Sweat is highly situational
Social context influences self-perception
Temperature shifts amplify sweat perception
Sweat patterns are not always logical
Wind and airflow regulate my sweat perception
Sweat regulation tools provide me psychological comfort
Language barriers influence my sweat response
Shifting focus reduces my sweat awareness
Documenting the process reshaped my perception (I often felt sweat more intensely than it actually appeared)
Sweat is difficult to quantify (It evaporates, gets absorbed by clothing, or is wiped away)
This intervention documents my attempts, challenges, and insights from collecting sweat through various methods, while also reflecting on my personal journey from discomfort to acceptance in sharing my project openly with others.
For the first experiment, I wrapped my torso and armpits with paper towels and covered them with plastic wrap to trap the sweat and prevent evaporation. This setup also amplified sweating due to the heat it generated. I wore several layers of sweatshirts, a coat, and cycled on a stationary bike outdoors for 45 minutes. I also used paper towels to collect sweat from my head, placing them in a zip-lock bag once saturated.
With help from my cousin and aunt, I removed the layers after exercising. Despite sweating profusely, especially on my shoulders, neck, and back, I could only extract a few milliliters of sweat by squeezing the soaked paper towels into a zip-lock bag. The paper towels absorbed too much sweat, making it difficult to retrieve, and possibly contaminated the liquid with fibers. I even tried using a salad spinner to centrifuge the paper towels, but it yielded little to no sweat. This method proved inefficient, highlighting the challenge of using absorbent materials for sweat collection.
I also attempted using underarm sweat pads, which I had used in the past to prevent sweat stains on my clothes (these pads absorb sweat well but become saturated quickly and detach, making them unreliable). While they collected some sweat, squeezing them was just as challenging as with paper towels, and the amount of liquid retrieved was minimal.
I realized that most absorbent materials would face the same issue: they trap sweat effectively but are difficult to extract from
For the second experiment, I aimed to collect sweat directly in liquid form without absorption. I created a suit from a large trash bag, sealing the openings at my waist, wrists, and neck with duct tape to prevent leaks. The black bag created a greenhouse effect, making me sweat even before exercise. I ran 15 minutes to the gym and attended a 45-minute body combat class.
However, issues arose quickly. Sweat caused the duct tape at my waist to loosen, leading to leaks. Additional tape provided temporary fixes, but continuous movement and excessive sweating overwhelmed the suit. By the end of the class, my shorts and lower shirt were soaked, and the bag had leaked significantly. Although I collected some sweat by placing the entire bag in a zip-lock and squeezing it, the quantity was even less than the first attempt. However, I noticed that sweat on plastic surfaces could be centrifuged for better extraction compared to absorbent materials.
I learned that collecting sweat during intense physical activity is complicated due to movement, leakage, and the difficulty of creating a perfect seal.
Recognizing that I sweat heavily in hot environments, I turned to the sauna at my gym. My first attempt involved collecting dripping sweat with a zip-lock bag by catching individual droplets. Although tedious and time-consuming, I managed to collect a small amount. Inspired by this, I devised a third experiment using a large trash bag in the sauna.
This time, I did not wash my body beforehand, unlike the first two experiments where I rinsed with water to avoid contamination. I had already exercised and was sweating, hoping this would yield more sweat. Entering the sauna with a trash bag was more conspicuous, but I explained to others that it was for an artistic project. Surprisingly, the response was positive, with people encouraging me and showing interest.
I experimented with different positions inside the bag: kneeling (uncomfortable but effective), standing (challenging to manage the bag), and finally sitting on the sauna bench with my legs inside the bag and torso bent forward to let sweat drip in. Sitting was the most comfortable and effective position for extended collection. After 20 minutes, feeling exhausted and overheated despite drinking water, I ended the session. The sweat collected filled the zip-lock bag about five times more than in my first experiment. However, I noticed tiny particles and lint in the liquid, likely from my dirty feet or the trash bag itself.
One of the most profound aspects of this intervention has been my personal journey with sweat and public perception. At home, exercising alone didn’t make me feel strange or nervous, though I had to open up to my family (my aunt and cousin) to help me and be close to my sweat, which was a personal barrier to overcome. Even at the gym, wearing layers to induce sweat made me feel out of place and awkward, conscious of how others might perceive me.
However, my time in the sauna marked a significant shift: instead of hiding or fabricating reasons for my actions, I openly shared my project with others, and to my surprise, their curiosity and support made me feel accepted.
Sharing my work not only reduced my own anxieties but also sparked genuine interest in others, encouraging conversations about sweat, the body, and material experimentation.
Sauna sessions are more efficient for collecting sweat than physical exercise, as they allow for steady sweat production without movement.
Absorbent materials like paper towels and underarm pads trap sweat but yield little liquid when squeezed. Plastic surfaces like trash bags are more efficient for sweat collection, especially when centrifuged.
Washing the body (only with water) beforehand helps avoid contamination, especially the feet, which introduced particles in my last experiment.
Long sessions are crucial but challenging. Hydration is essential, and taking breaks is difficult due to mobility constraints.
A larger bag would improve comfort and coverage. Switching from shorts to a minimal slip would reduce sweat absorption by clothing. Washing the bag before use would help eliminate particles.
Embracing the discomfort of sweating publicly and sharing my project openly has been a transformative experience, leading to more authentic interactions and personal growth.
Exploring other materials like cellulose sponges that might absorb and release sweat more efficiently is a potential next step. Additionally, improving the design of sweat collection suits for better mobility and seal could enhance future collections.
In this intervention, I explore the potential of sweat as a valuable material, challenging its perception as a mere bodily byproduct. I brainstormed 30 ideas for using sweat, including rehydration, watering plants, pH indicators, lens solution, conductive liquid, crystal formation, salt extraction, fragrance, lubricant, health monitoring, altering sound circuits, ink, mycelium nutrition, conductive sensors, makeup remover, dental rinse, circuit coolant, fermentation and cooking, screen cleaner, clay molding, stress measurement with GSR sensors, natural exfoliant, galvanic cell electrolyte, jewelry cleaner, mineral supplement, garment steaming, and vapor inhalation.
While some of these ideas may seem impractical, they prompt reflection on utilizing the human body as a resource in extreme scenarios, such as space travel. With the average human body producing approximately 1,044 liters of sweat annually, it remains an underexplored liquid with unique properties.
From this list, I am currently focusing on two ideas with the most potential: using sweat as a pH-reactive material for color changes and exploring the crystallization of sweat.
Bromothymol Blue (BTB) was selected due to its color change range (yellow at pH <6, green at neutral, blue at pH >7.6), which aligns with sweat’s natural pH fluctuations. It was purchased in powdered form and dissolved in alcohol, as it is not water-soluble. The concentrated solution reacted strongly when tested with vinegar and baking soda, confirming its sensitivity.
Despite shrinkage, dried agar samples worked as a reusable pH-sensitive canvas, able to change color repeatedly when exposed to different pH liquids without degrading. The color shift was vivid and noticeable, making it a promising interactive material.
To prevent shrinkage, I made a larger sample (400ml water, 16g agar, 40ml glycerin), but it broke into pieces as it dried. This was a recurring issue, possibly due to insufficient agar or glycerin. Additionally, the sample turned yellow just from air exposure, showing sensitivity even without direct liquid contact.
Over time, agar pieces stabilized into a leather-like texture, though results were inconsistent. The material absorbed water quickly, making it highly responsive to pH but also prone to leaving traces on the skin. Its extreme moisture sensitivity made it difficult to control.
Alginate was more promising due to its transparency and structural stability. The new recipe (400ml water, 15g alginate, 30g glycerin) varied in BTB concentration, producing darker shades with higher amounts. Samples took around three days to dry in well-ventilated conditions.
Once dried, alginate had a soft, translucent, skin-like feel, much lighter and more aesthetically pleasing than agar. It reacted to pH changes from sweat and vinegar, but the transition was slower, likely due to lower liquid absorption.
When exposed to direct liquid sweat, alginate changed color but also disintegrated, forming holes where the liquid sat. This suggested it was not ideal for prolonged exposure to moisture, limiting its application. Modifying the formula or adding a stabilizing component could help prevent this issue.
Paper absorbed the solution well, allowing for multi-color painting effects with different pH liquids. However, the colors faded quickly. Applying it with my hand stained my skin for two days, though BTB is non-toxic. On textiles (100% cotton), diluted BTB in water did not stain, but direct application with alcohol resulted in a stable, reactive fabric.
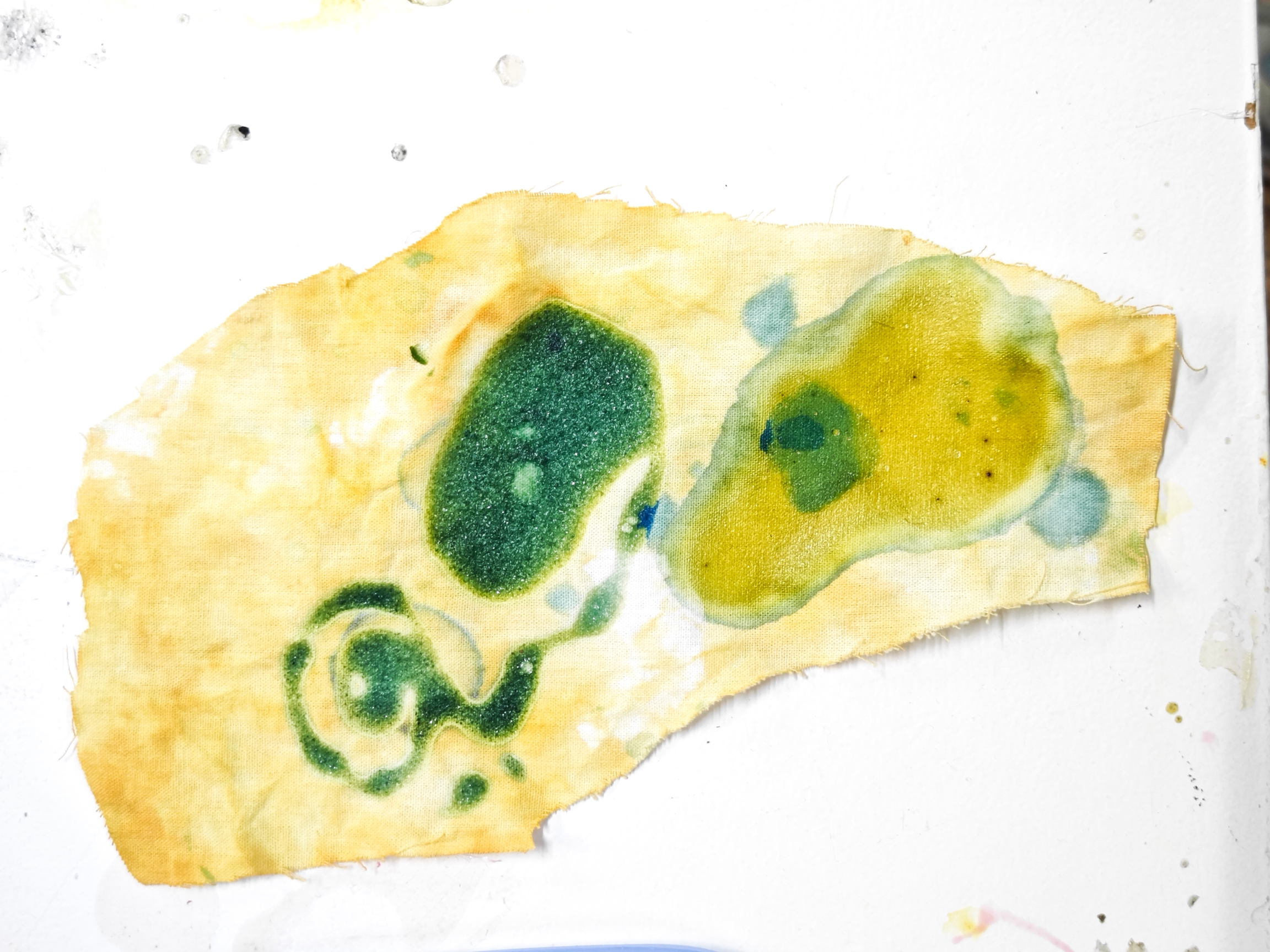
I tested direct dyeing with BTB dissolved in alcohol on a 96% cotton T-shirt, applying it in an abstract pattern instead of full immersion to save material. The orange hue adhered well, with no noticeable transfer or strong odor. When sweat was applied, the color reacted to a deep blue-green, likely due to the high BTB concentration, ensuring a strong contrast but not so colorful.
To test real-world reactivity, I wore the shirt to a crowded nightclub. As expected, only the armpits sweated enough to trigger a reaction, turning slightly blue-green. However, cotton absorbed sweat slowly, requiring heavy moisture for a visible change. The dye also bled slightly, suggesting a more absorbent or treated fabric could enhance results.
To mimic salt crystal formation, I tested a supersaturated solution method, where saltwater is heated, saturated, cooled, and left with a suspended object for slow crystal growth. I replaced saltwater with concentrated sweat, though this process requires weeks or months for results.
To speed up evaporation, I heated sweat instead of air-drying it. This produced less salt, which was dark and impure, likely due to natural sweat components and contaminants. The strong odor intensified in the crystallized residue, making this method unpleasant.
Observing the crystallization process, I noted that rapid temperature shifts (heating or cooling too fast) led to poor crystal formation, while slow, controlled evaporation encouraged better-defined structures. A balance is needed between time efficiency and crystal quality.
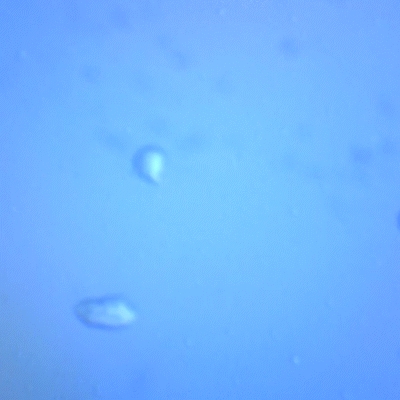
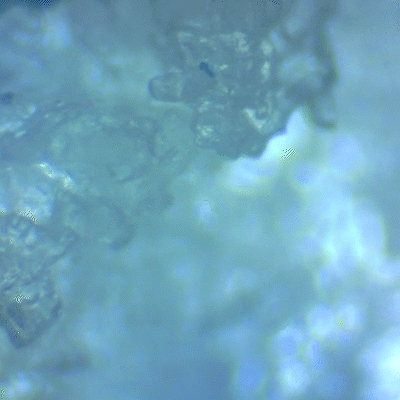
Using a microscope, I examined the crystallized sweat. While bacteria or microorganisms were not visible due to limited magnification, crystal structures were clearly present. Comparing sweat salt vs. purified salt, the sweat-derived sample was significantly darker and contained more impurities, confirming the challenge of obtaining clean sweat crystals.
Despite shrinkage, dried agar maintained repeated color change without degrading, proving its potential as an interactive material. However, its high moisture sensitivity led to structural instability and staining.
Alginate samples provided better transparency and structure than agar, resembling skin-like textures. However, prolonged exposure to liquid caused disintegration, limiting its usability.
For BTB to bond with fabric, a high concentration was required, resulting in dark, intense colors. Additionally, fabric needed to be heavily moistened for visible pH changes, which caused the dye to bleed.
Evaporating sweat (ambient conditions) resulted in small, impure salt formations due to contaminants.
Rapid sweat evaporation through heat application resulted in dirty and impure salt formations. The process also produced a strong odor, and the crystallized residue was noticeably darker and less structured.
Experiment with a combination of agar and alginate to balance agar’s flexibility and pH reactivity with alginate’s stability and aesthetics.
Create a diluted BTB solution for more even staining and test it in different fabric areas to compare reactivity and color variations. Additionally, test the effect of mordants on BTB fixation.
Collect and purify sweat samples before crystallization to reduce impurities. Perform controlled heating tests at different intensities to find the optimal balance between time and crystal definition.
Conduct a detailed biochemical analysis of sweat components to identify dominant compounds and explore new application possibilities based on its unique composition.
fffffffç
Freshly collected sweat is surprisingly odorless, carrying only a faint scent reminiscent of the sauna environment. However, when stored at room temperature, it darkens to a reddish hue within days, and the odor intensifies, becoming unpleasant. Refrigeration slows this transformation but does not prevent it entirely, suggesting that freezing may be necessary for long-term preservation. Understanding how sweat breaks down over time could be crucial for future experiments.
Once again, engaging with others in the sauna proved to be an enriching experience. This time, I met a couple of professors who showed genuine curiosity about my project. We had a deep conversation about sweat, its perception, and its potential applications, and they even offered to help record future experiments. These moments reinforce how openly discussing my research shifts perspectives and creates meaningful exchanges.
Sweat rate is a useful metric to understand how much fluid your body loses during physical activity. It helps determine proper hydration strategies and can vary significantly based on intensity, duration, and environmental conditions.
To compare sweat production under different conditions, I performed:
Body Pump session (45 min) – A full-body workout involving resistance training with weights.
Sauna session (20 min) – Passive sweating due to heat exposure.
Pre-exercise measurements
Empty bladder.
Weigh yourself naked (A).
Weigh your water bottle before the activity (X).
Activity phase
Perform the chosen exercise while tracking time.
Drink water as needed.
Post-exercise measurements
Dry off any sweat completely.
Weigh yourself naked again (B).
Weigh the remaining water in the bottle (Y).
Calculations
Weight lost during exercise = A - B (C)
Fluid intake during exercise = X - Y (Z)
Total sweat loss = C + Z
Sweat rate = (C + Z) / exercise duration (converted to liters per hour)
My calculated sweat rates are 1.07 L/h during a 45-minute body pump session and 1.67 L/h during a 20-minute sauna session. These rates fall within the typical range of 0.5–2.0 L/h observed during physical activity.
Hyperhidrosis often leads to excessive sweating even without triggers like heat or exercise. However, my sweat rates during these activities don't appear abnormally high. It's important to note that factors such as measurement inaccuracies and environmental conditions can influence sweat rate calculations.
Lab de Vic
Mapping my sweat
To explore variations in sweat pH across my body and different activities, I used Metria Universal Indicator Paper (pH 1-14). According to the instructions, the strip must be in contact with sweat for 1-2 seconds, then shaken to remove excess liquid and compared to the color chart within 3-5 seconds before fading.
I mapped my sweat pH after two activities:
Body Pump (45 min)
Sauna (20 min) [Post-Body Pump]
Right after each activity, I applied pH strips to six areas: Forehead, chest, armpits, elbows, lower back, and behind the knees.
The average pH of human sweat typically ranges between 4.2 and 5.5, contributing to the skin’s natural acidity. However, in areas like the armpits and scalp, where apocrine glands are present, the pH can be higher, reaching 6.0 to 7.5.
In my measurements, armpit sweat during Body Pump reached pH 8, which is slightly above the usual range. This aligns with the presence of apocrine sweat, which contains more proteins and lipids, potentially raising pH. Conversely, sweat from areas like elbows and knees remained around pH 5-6, aligning with typical eccrine sweat values.
The sauna session resulted in an overall increase in sweat pH, except in the armpits. This shift could be due to prolonged sweating, dilution effects, or metabolic factors, suggesting that later-stage sweat tends to be more basic.
Beyond testing fresh sweat directly on my body with pH indicator strips, I experimented with how my collected sweat reacted to Bromothymol Blue (BTB) solutions at different pH levels. I used:
BTB blue solution (~pH 8)
BTB yellow solution (~pH 3)
Surprisingly, when adding sweat samples from different collection methods—older, more contaminated sauna sweat; recently collected, cleaner sauna sweat; and fresh sweat from exercise—none caused a visible color change in either BTB solution.
To push the experiment further, I tested highly concentrated BTB dissolved in alcohol (which had an intense orange-yellow hue). This time, adding sweat did trigger color changes, turning blue (~pH 7-8) across all samples. Interestingly, the oldest sauna sweat produced the fastest and most intense color shift, suggesting that stored sweat might become more basic over time.
However, this experiment alone isn't enough to confirm pH shifts in aged sweat. Next steps include re-testing different sweat samples using pH strips to cross-verify results and better understand potential pH changes over time.
I first tested pure gelatin mixed with BTB, but once dried, the material did not absorb any liquid, preventing pH-based color changes. This suggested gelatin alone was not suitable for this application.
Since agar and alginate each had useful properties, I attempted a 50/50 and 70/30 mix to combine their strengths. However, their different preparation methods posed a challenge: agar requires heat, while alginate is prepared cold.
When adding alginate to the hot mixture, it did not dissolve well, requiring blending for a uniform texture. The resulting material combined properties from both, but it still fractured, suggesting alginate was not the best stabilizer for preventing cracks.
To improve structural integrity, I tested xanthan gum, a strong gelling agent. The recipe used was:
Ingredient
Amount
Water
400ml
Agar
16g
Glycerin
40ml
Xanthan Gum
1g
This resulted in a much stronger structure that did not break, but the texture was too thick and less reactive to pH changes compared to pure agar.
Next, I tried mixing agar and gelatin, as both require hot preparation, making the process easier. The recipe used was:
Ingredient
Amount
Water
200ml
Agar
8g
Gelatin
8g
Glycerin
20ml
The resulting samples were colorful, highly reactive to pH, and had a great texture: flexible, resistant, and not too thick. This mix showed the most promising results so far.
As I progressed, I poured the hot biomaterial mixtures directly onto textiles to observe adhesion and flexibility upon drying. The biomaterial bonded well to the fabric while retaining its pH reactivity, showing potential for sweat-responsive wearable applications.
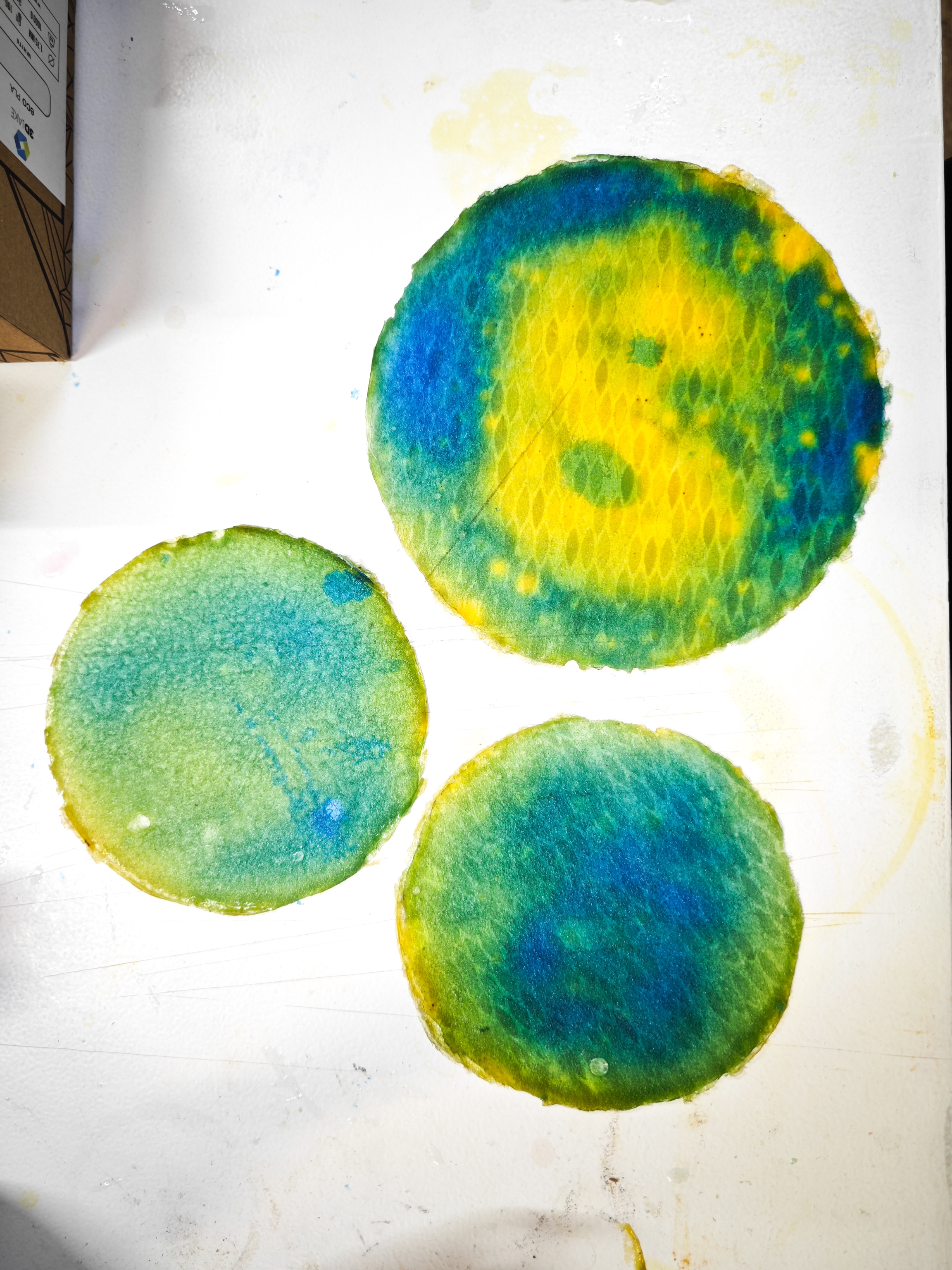
Testing pH reactivity on a yellow-dyed fabric revealed that sweat changed the material to a greenish hue almost instantly. However, the color was not very saturated. This suggests that finding the right fabric type and BTB concentration is crucial for achieving a stronger, more noticeable reaction, as cotton may not be the most reactive textile.
I re-dyed the entire cotton T-shirt by submerging it in a BTB solution (alcohol + water) for a full day. Once dried, an unexpected pattern appeared, previous sweat-marked areas remained visible, resembling stains left by perspiration. The color remained an intense orange, and I wore the shirt again for the ELISAVA WIP exhibition.
This time, after prolonged use, I sweated more in the armpits, but the color shift was barely noticeable, appearing more like a slightly darker damp stain rather than a vivid pH reaction. However, after removing the shirt, I found that the dye had transferred onto my skin, particularly under my armpits, turning them yellow.
Possible next steps:
Changing the base color: Testing if rinsing the shirt in tap water alters the starting hue, potentially making pH shifts more visible.
Applying a mordant: To improve dye fixation and reduce transfer to the skin.
Testing wash resistance: Seeing how the dye reacts to washing and whether it remains stable.
I performed another rapid sweat evaporation experiment using a pot and heat, but this time, instead of applying high heat immediately, I used a gradual heating approach to better control the evaporation process. The resulting salt was still impure, with a yellow-green tint, but showed improvement compared to my previous attempt, where the salt was darker and more contaminated. This likely resulted from using a cleaner sweat sample and a slower crystallization process.
However, to further refine the results, I need to switch to a new, unused pot, as the current one is worn out and stained from repeated use.
sulfato de cobre
ffff
vvv
fragances? with activated carbon
cheese??
Collecting sweat is a critical step in my project, enabling me to experiment with sweat as a material. Inspired by artist Alice Potts, who highlighted in a how challenging it is to collect enough sweat for her crystalized garments, I realized that gathering sweat in sufficient quantities is one of the biggest hurdles. I also explored where athletes shared methods to collect sweat for hydration calculations, which helped me design my own techniques.
In the , I first incorporated BTB into an alginate mixture, but the material took too long to dry. Switching to agar, I added BTB powder (without alcohol) when the mixture was still warm. The agar reacted well to pH changes once dried, but over time, it shrank and often remained soft, possibly due to BTB concentration or residual alcohol.
Inspired by , I aimed to crystallize sweat and extract its solid minerals. I started by evaporating sweat on a plastic lid, adding layers over time. However, since the sweat contained impurities, the crystals formed around dirt particles, preventing the growth of large salt formations.
Here is a h and a for salt crystals.
Here is a of sweat fractals crystal formation using a microscope.
Building on my , I refined my sweat collection process using the trash bag sauna method, which remains the most effective technique. Sitting inside a sealed trash bag in the sauna for 20 minutes, I managed to collect ~200 mL of sweat from my entire body.
To prevent contamination, I covered my feet with sandwich bags, which alone yielded ~50 mL of sweat. Hydration was crucial during this process to prevent dizziness. Given that my total sweat loss in the sauna was 0.55 kg (refer to ), this means I successfully recovered 36.36% of my lost sweat, a significant improvement in collection efficiency. The sweat was immediately stored in an airtight zip-lock bag to minimize evaporation and contamination.
Sweat reacts to exercise, heat, stress, and relaxation, varying in location, intensity, and triggers such as temperature, clothing, and emotions. Understanding these patterns helps analyze how the body cools itself and adapts to different conditions.
Sweating influences self-image and emotions, often associated with discomfort, relief, or transformation. Perception changes depending on whether I am alone or in public, and experimenting with sweat can reshape my relationship with it.
Social norms dictate where and when sweat is acceptable or shameful, with differences in gender, cultural perception, and marketing influence. Exploring these perspectives helps uncover how sweat is controlled or stigmatized.
Sweat, often seen as waste, has potential applications in biomaterials, art, and science. Investigating its properties and cultural significance opens possibilities for sustainability, reuse, and creative experimentation.